MORBUS KRABBE
Description
The infantile form has an onset at 2-6 months of age and is divided into 3 stages. In the first stage, symptoms include irritability, stiffness, poor head control, feeding difficulties, intermittent thumb clasp, episodes of increased temperature, and developmental delay. In the second stage, hypertonic episodes occur with opisthotonus, myoclonic seizures, developmental regression, fisting and vision deficits. In the third stage, hypotonia, blindness and deafness occur. Patients progress into a vegetative state and die before the age of 2-3 years, generally due to respiratory infections. In the late infantile/juvenile (1-8 years) and adult (>8 years) forms, the presenting symptoms vary greatly and progression is variable (generally slower in older patients). Patients with late infantile /juvenile onset most resemble infantile patients, while the first signs in adult forms are often weakness, gait disturbances (spastic paraparesis or ataxia), burning paresthesias, hemiplegia, and/or vision loss, with or without peripheral neuropathy. Cognitive regression is variable and often absent in adult forms.
Etiology
The disease is due to mutations in the GALC gene (14q31) encoding the lysosomal enzyme galactocerebrosidase, that catabolizes the hydrolysis of galactose from galactocerebroside and galactosylsphingosine (psychosine). The accumulation of cytotoxic psychosine leads to apoptosis of oligodendrocytes and demyelination of the CNS and PNS. Rarely, infantile Krabbe disease is caused by a mutation in the prosaposin (PSAP) gene (10q21-q22), encoding sphingolipid activator protein saposin-A, necessary for GALC activity.
Diagnostic methods
Diagnosis is suspected by the clinical picture, slow nerve conduction velocity, abnormal electroencephalogram, and brain MRI revealing white matter abnormalities (demyelination, gliosis, late-stage cerebral atrophy, cerebral calcifications). It is established from enzymatic assays in leukocytes or in cultured fibroblasts that reveal, in almost all cases, the GALC deficiency. Histologically, characteristic globoid cells (often multinucleated cells derived from macrophages that contain non-hydrolyzed galactocerebroside) are present in the white matter. Mutation analysis confirms the diagnosis.
Differential diagnosis
Differential diagnosis includes metachromatic leukodystrophy, GM1 gangliosidosis, GM2 gangliosidosis, Canavan disease, encephalopathy due to prosaposin deficiency, X-linked adrenoleukodystrophy, Pelizaeus-Merzbacher disease and Alexander disease (see these terms).
Antenatal diagnosis
Antenatal diagnosis (enzymatic assay or mutation analysis) is possible for at-risk families. If the disease-causing mutations in the family are known, pre-implantation genetic diagnosis is possible.
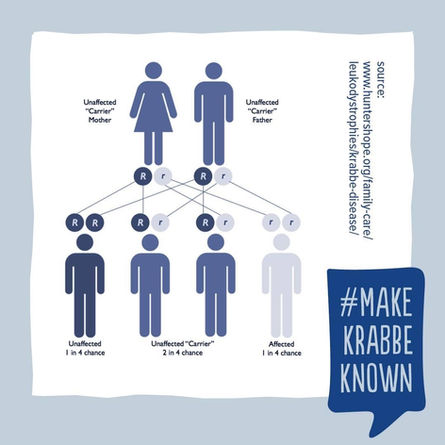
Genetic counseling
Transmission is autosomal recessive. Genetic counseling should be offered to at-risk couples (both individuals are carriers of a disease-causing mutation) informing them of the 25% chance of having an affected child.
Management and treatment
Treatment is limited to hematopoietic stem cell transplantation in pre-symptomatic infantile patients and mildly affected late-onset patients and has been shown to slow the progression of the disease. Other treatment options (i.e. chaperone therapy, enzyme replacement therapy, gene therapy) are currently under investigation in animal models.
Prognosis
Neurodegeneration and early death (< 2-3 years) occurs in most infantile cases. In late infantile/juvenile patients, the disease is generally fatal 2-7 years after the symptoms begin. Adult-onset patients can survive many years after symptoms present.